Difference between revisions of "Lighting Technologies"
***** (***** | *****) |
***** (***** | *****) m |
||
| Line 190: | Line 190: | ||
|} | |} | ||
</div> | </div> | ||
| + | <br> | ||
| + | |||
| + | = <span><font size="3">1</font><span> </span></span><font size="3">Lighting Technologies</font> = | ||
| + | |||
| + | Artificial light is produced by the user at the expense of some energy and it can be classified as a) flame-based lighting and b) electricity-based lighting. | ||
| + | |||
| + | | ||
| + | |||
| + | | ||
| + | |||
| + | == <span><span><font size="3">1.1</font> </span><span><font size="3"> </font></span></span><span><span><font size="3">Flame-based Lighting</font></span></span> == | ||
| + | |||
| + | ''' ''' | ||
| + | |||
| + | Flame-bases lighting is related to the production of light from fire. Burning of carbon-based fuels such as wood, kerosene, vegetable oil, gas, wax, etc., to produce light is based on the principle of ''‘incandescence’''. Incandescent lamps in general and flame-based lighting in particular are not very energy-efficient, as most energy is lost in the form of waste heat. Furthermore flame-based lighting results in the production of unwanted pollutants, which can be harmful to health. | ||
| + | |||
| + | Gas lamps or kerosene pressure lamps with an incandescent mantle are more efficient than candles or simple kerosene lamps. In this case the light is not emitted directly by the flame but by the bright glowing mantel made of heat resistant fibres. But the disadvantage of heat and pollution is similar. | ||
| + | |||
| + | ''' ''' | ||
| + | |||
| + | == <span><span><font size="3">1.2</font> </span><span><font size="3"> </font></span></span><span><span><font size="3">Electricity-based Lighting</font></span></span> == | ||
| + | |||
| + | Lamps that produce light, using electricity have now become the standard for modern lighting. Existing lamps can be categorised as detailed below. | ||
| + | |||
| + | === <span><span><span>''<font size="3">1.2.1</font>''<span> </span></span>''<font size="3">Incandescent Lamps</font>''</span></span> === | ||
| + | |||
| + | Incandescent lamps are based on the principle of incandescence, where a filament is heated to produce light, such as in standard tungsten filament lamps. Their energy efficiency is comparably low. For instance, when a typical 100 W incandescent lamp is lit, only about 10 W of energy is converted to visible light, the rest is converted to waste heat. | ||
| + | |||
| + | An improved type, namely, the Halogen lamps are high pressure, incandescent lamps that consist of a tungsten filament inside a quartz envelope, which contains halogen gases such as iodine and bromine that allow filaments to work at higher temperatures and higher efficiencies. | ||
| + | |||
| + | In halogen lamps, the quartz envelope is closer to the filament than the glass used in conventional light bulbs. Heating the filament to a high temperature causes the tungsten atoms to evaporate and combine with the halogen gas. These heavier molecules are then deposited back on the filament surface. This recycling process increases the life of the tungsten filament and enables the halogen lamp to produce more light per unit of energy. Consequently, halogen lamps are used in a variety of applications, including automobile headlights. Halogen lamps that work on both A.C. and D.C. power, ranging from 6 V to 230 V are available today. But usually, these lamps get very hot while in operation. They are sensitive to voltage fluctuations. Some studies indicate that their life expectancy is decreased to 50% by 5% overvoltage (e.g.: 0.6V on 12V) and by about 75% by 10% overvoltage. | ||
| + | |||
| + | === <span><span><span>''<font size="3">1.2.2</font>''<span> </span></span>''<font size="3">Gas Discharge Lamps</font>''</span></span> === | ||
| + | |||
| + | Gas Discharge Lamps are based on a glowing gas in a glass enclosure. Examples for this type are sodium, mercury vapour and mercury tungsten (blended) lamps. | ||
| + | |||
| + | In these lamps, the atoms or molecules of a gas inside a glass, quartz, or translucent ceramic tube, are ionized by an electric current sent through the gas or by a radio frequency or microwave field in proximity to the tube. This results in the generation of light - usually either visible or ultraviolet (UV). The colour depends on both the mixture of gasses and other materials inside the tube as well as the pressure and type and amount of the electric current or RF power (Radio-frequency power). There are a variety of gas discharge lamps, which are available in different forms, as explained further on. | ||
| + | |||
| + | ==== <span>1.2.2.1 </span>Fluorescent Lamps ==== | ||
| + | |||
| + | Fluorescent lamps are a special class of gas discharge lamps. Their functioning relies on the <span>principle of fluorescence: Inside the glass tube is a partial vacuum and a small amount of mercury. An electric discharge in the tube causes the mercury atoms to emit light. The emitted light is in the ultraviolet range and is invisible, and also harmful to living organisms, so the tube is lined with a coating of a fluorescent (phosphoric) material, which absorbs the </span><acronym>UV</acronym><span> and re-emits visible light. </span> | ||
| + | |||
| + | <span>They are sensitive to the ambient temperature around them. 1% loss in light output can be expected for every 2<sup>o </sup>F (1.1<sup>o </sup>C) above the optimum ambient temperature of 76<sup>o </sup>F (25<sup>o</sup> C), in most of the fluorescent lamps. But they are definitely more efficient than the incandescent lamps. </span>Hum and flicker might be a problem in some cases. Frequent switching on and off will reduce the life of a fluorescent lamp. | ||
| + | |||
| + | ==== <span>1.2.2.2 </span>Compact Fluorescent Lamps (CFLs) ==== | ||
| + | |||
| + | A CFL can be seen as an advanced version of a fluorescent lamp. The salient features of it are: It consists of a gas-filled glass tube with two electrodes mounted in an end cap. It contains a low-pressure mix of argon gas, mercury vapour, and liquid mercury, and is coated on the inside with three different phosphorous substances. The electrodes provide a stream of electrons to the lamp and the ballast controls the current and voltage flowing into the assembly. The ballast, in general an electronic circuit, may be attached directly to the lamp, or may be remotely connected. | ||
| + | |||
| + | CFLs are compact and are ideal for use in homes, work areas, schools, workshops, etc. They are more energy-efficient than incandescent light bulbs using between one third and one fifth of the energy. They are sensitive to the ambient temperatures, just like the standard fluorescent lamps. | ||
| + | |||
| + | The life of a CFL is significantly shorter if it is used only for a few minutes at a time. Lab tests demonstrated that lifespan can be reduced down to 15% in the case of a 5 minute on/off cycle. | ||
| + | |||
| + | ==== <span>1.2.2.3 </span>Low Pressure Sodium Lamps ==== | ||
| + | |||
| + | A low pressure sodium lamp consists of a tube made of special sodium-resistant glass containing sodium and a neon-argon gas mixture. These lamps usually require 5 to 10 minutes to warm up. This light is basically monochromatic orange-yellow. This monochromatic light causes a dramatic lack of colour rendition: everything comes out in an orange-yellow version of black and white. Hence, low pressure sodium lamps are not suitable for use in homes, offices, workshops, etc. But they are widely used for street lighting purposes. | ||
| + | |||
| + | Low pressure sodium lamps are the most energy efficient visible light sources in common use. These lamps have luminous efficacies as high as 180 lumens per Watt, whereas a typical incandescent lamp has around 12 lumens per Watt and a standard fluorescent lamp has around 45 lumens per Watt. | ||
| + | |||
| + | | ||
| + | |||
| + | === <span>''<font size="3">1.2.3</font>''<span> </span></span>''<font size="3">Light Emitting Diodes (LEDs)</font>'' === | ||
| + | |||
| + | LEDs are semiconductor devices similar to p-n junction diodes, specially engineered to emit visible light of a particular wavelength, giving out a specific colour. | ||
| + | |||
| + | On supplying electrical power to an LED, electrons are made to “fall” from a high energy level to a low energy level inside the semiconductor material, releasing some energy, which is perceived as <span>visible light. </span> | ||
| + | |||
| + | LEDs are usually monochromatic and are very energy-efficient and therefore used extensively as indicator lamps on many electronic devices. Red, green, and orange LEDs as indicator lamps are usually well known. They are usually operated with no more than 20 – 70 mW of electrical power and not adequate for lighting purposes. However, the development of bright white LEDs advanced rapidly in recent years and is achieving fast access to the market. In 1999, the first 1 W white LEDs came on the market. In 2002, first 5 W models were available. | ||
| + | |||
| + | Parallel to this augmentation of the total power there has been also a dramatic increase in the luminous efficacies (see chapter 3.3) of LEDs during the past few years. Today the LEDs attain similar efficacy as CFLs and it is projected to increase further. In the last years, the price of bright white LEDs has fallen drastically and their availability increased considerably. Hence, LEDs are now used in many appliances like torches, bicycles and car lighting and are considered more and more for main stream lighting purposes. | ||
| + | |||
| + | LEDs have a longer life than the incandescent lamps and all types of gas discharge lamps. As they are solid-state devices, theoretically they offer operating periods of 100,000 to 150,000 hours. But they are the same time sensitive to ambient temperatures what caused some problems in first projects on LEDs as lighting devices in the tropics. A study by Lumitex Inc., U.S.A, indicates a substantial fall in the life of an LED, at elevated ambient temperatures (Fig. 4). Hence, special care must be taken to house LEDs properly, to ensure expected life. Life here implies, the time taken for the light output to fall to its 50% value, when compared to its initial light output. | ||
Revision as of 13:00, 18 May 2009
Dishna Schwarz
Elmar Dimpl
George C. Bandlamudi
Michael Blunck
updated by E. Dimpl, May 2009
1 Introduction
The rural poor in most developing countries still lack access to basic energy services and are therefore often incapacitated to afford good lighting. It is worthwhile to examine and consider lighting methods that would be both economical in terms of energy consumption and cost, to be successfully disseminated amongst this target group. This paper will give a brief theoretical background on physical aspects of light and frequently used terminologies and physical units (chapter 2), describe and compare different technological options for lighting (chapter 3) and present a useful method for appropriate lighting design (chapter 4).
2 Some Facts about Light
2.1 Electromagnetic Radiation and the Visible Spectrum
The electromagnetic spectrum implies different types of radiation, ranging from high-energy gamma-rays to low-energy radio waves. However, the human eye is sensitive only to radiation with wavelengths in the range of 0.38 to 0.76 micrometre, which we call ‘the visible spectrum’. The electromagnetic wave spectrum is illustrated in Fig.1.
Radiation is the cause and the visible light is the result. It takes a certain amount of energy to produce a given amount of light.
Light of one fixed wavelength is termed as ‘monochromatic’ light, characterised by its corresponding colour at that wavelength. For instance, if a lamp is producing only radiation of 0.555 micrometre, then the resulting light is monochromatic, with its yellow-green colour. Sensitivity of the human eye is 100% at a wavelength of 0.555 micrometre. Light perceived as white is a mixture of light intensity across the visible spectrum. In display and lighting technology the impression of white light is often created by mixing appropriate intensities of different colours like red, green and blue etc. The number of combinations of light wavelengths that produce this sensation of white light is practically infinite.
Fig.1: The electromagnetic wave spectrum
2.2 Lighting Terminology
2.2.1 Luminous Intensity
Luminous intensity is a measure of the amount of light originated from the source, its light output, the unit of which is the candela (cd).
2.2.2 Luminance
Luminance is a measure of the brightness of a particular surface if considered as a large light source. A common unit of luminance is cd/m².
2.2.3 Luminous Flux
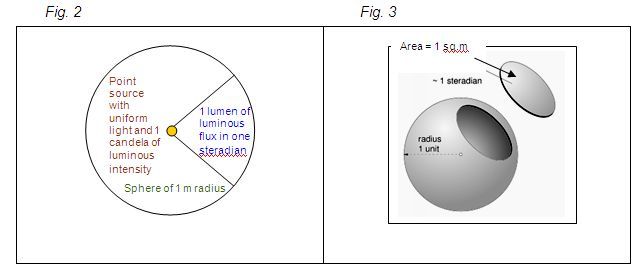
Luminous flux (or luminous power) is the quantity of light energy emitted in all directions. The unit of luminous flux is lumen (lm). One lumen is the luminous flux of the uniform point light source that has luminous intensity of 1 candela (cd) and is contained in one unit of spatial angle (or 1 steradian).
Steradian is the spatial angle that limits the surface area of the sphere equal to the square of the radius. If the radius of the sphere is 1 metre, which implies its area to be 4πr², then the luminous flux of the point light source of 1 candela is 4π lumens, as shown in Figures 2 & 3.
For instance, a light source can be emitting light with an intensity of one candela in all directions, or one candela in just a narrow beam (as in most LEDs). The intensity is the same but the total energy flux from the lamp, in lumens, is not the same. The output from a lamp is usually quoted in lumens, summed over all directions, together with the distribution diagram in candela.
1.1.1 Illuminance
Illumincance is a measure of the amount of light falling on a particular surface. Its unit is lux (lx), defined as equal to one lumen per metre squared (1 lm/m²). LUX meters are available in the market, which could be used by a lighting designer to measure the light falling on a given surface.
Repetitive summary:
- The intensity of a light source is measured in candelas;
- The total light flux in transit is measured in lumens (1 lumen = 1 candela X steradian);
- The amount of light received per unit of surface area is measured in lux (1 lux = 1 lumen/square meter).
Having considered the various aspects related to lighting terminology, it is important to get an impression about the typical lighting levels that are common in day to day life, as shown in Tab.1.
Tab.1: Typical lighting levels in day to day life
|
Outdoor |
Illuminance (lux) |
|
Bright sun |
50,000 – 100,000 |
|
Hazy day |
10,000 – 50,000 |
|
Full moon |
0.05 - 0.2 |
|
Indoor |
Illuminance (lux) |
|
Office or workshop |
200 - 300 |
|
Reading Area |
300 - 500 |
|
Class Room |
300 |
|
Health Centres |
Illuminance (lux) |
|
Examination Area (Spot Light) |
500 |
|
Surgery Room (Spot Light) |
2000 |
|
Domestic Lighting |
Illuminance (lux) |
|
Living Room |
100 - 300 |
|
Kitchen Working Area |
300 |
|
Corridors |
50 - 100 |
|
Good street light |
20 |
1 Lighting Technologies
Artificial light is produced by the user at the expense of some energy and it can be classified as a) flame-based lighting and b) electricity-based lighting.
1.1 Flame-based Lighting
Flame-bases lighting is related to the production of light from fire. Burning of carbon-based fuels such as wood, kerosene, vegetable oil, gas, wax, etc., to produce light is based on the principle of ‘incandescence’. Incandescent lamps in general and flame-based lighting in particular are not very energy-efficient, as most energy is lost in the form of waste heat. Furthermore flame-based lighting results in the production of unwanted pollutants, which can be harmful to health.
Gas lamps or kerosene pressure lamps with an incandescent mantle are more efficient than candles or simple kerosene lamps. In this case the light is not emitted directly by the flame but by the bright glowing mantel made of heat resistant fibres. But the disadvantage of heat and pollution is similar.
1.2 Electricity-based Lighting
Lamps that produce light, using electricity have now become the standard for modern lighting. Existing lamps can be categorised as detailed below.
1.2.1 Incandescent Lamps
Incandescent lamps are based on the principle of incandescence, where a filament is heated to produce light, such as in standard tungsten filament lamps. Their energy efficiency is comparably low. For instance, when a typical 100 W incandescent lamp is lit, only about 10 W of energy is converted to visible light, the rest is converted to waste heat.
An improved type, namely, the Halogen lamps are high pressure, incandescent lamps that consist of a tungsten filament inside a quartz envelope, which contains halogen gases such as iodine and bromine that allow filaments to work at higher temperatures and higher efficiencies.
In halogen lamps, the quartz envelope is closer to the filament than the glass used in conventional light bulbs. Heating the filament to a high temperature causes the tungsten atoms to evaporate and combine with the halogen gas. These heavier molecules are then deposited back on the filament surface. This recycling process increases the life of the tungsten filament and enables the halogen lamp to produce more light per unit of energy. Consequently, halogen lamps are used in a variety of applications, including automobile headlights. Halogen lamps that work on both A.C. and D.C. power, ranging from 6 V to 230 V are available today. But usually, these lamps get very hot while in operation. They are sensitive to voltage fluctuations. Some studies indicate that their life expectancy is decreased to 50% by 5% overvoltage (e.g.: 0.6V on 12V) and by about 75% by 10% overvoltage.
1.2.2 Gas Discharge Lamps
Gas Discharge Lamps are based on a glowing gas in a glass enclosure. Examples for this type are sodium, mercury vapour and mercury tungsten (blended) lamps.
In these lamps, the atoms or molecules of a gas inside a glass, quartz, or translucent ceramic tube, are ionized by an electric current sent through the gas or by a radio frequency or microwave field in proximity to the tube. This results in the generation of light - usually either visible or ultraviolet (UV). The colour depends on both the mixture of gasses and other materials inside the tube as well as the pressure and type and amount of the electric current or RF power (Radio-frequency power). There are a variety of gas discharge lamps, which are available in different forms, as explained further on.
1.2.2.1 Fluorescent Lamps
Fluorescent lamps are a special class of gas discharge lamps. Their functioning relies on the principle of fluorescence: Inside the glass tube is a partial vacuum and a small amount of mercury. An electric discharge in the tube causes the mercury atoms to emit light. The emitted light is in the ultraviolet range and is invisible, and also harmful to living organisms, so the tube is lined with a coating of a fluorescent (phosphoric) material, which absorbs the <acronym>UV</acronym> and re-emits visible light.
They are sensitive to the ambient temperature around them. 1% loss in light output can be expected for every 2o F (1.1o C) above the optimum ambient temperature of 76o F (25o C), in most of the fluorescent lamps. But they are definitely more efficient than the incandescent lamps. Hum and flicker might be a problem in some cases. Frequent switching on and off will reduce the life of a fluorescent lamp.
1.2.2.2 Compact Fluorescent Lamps (CFLs)
A CFL can be seen as an advanced version of a fluorescent lamp. The salient features of it are: It consists of a gas-filled glass tube with two electrodes mounted in an end cap. It contains a low-pressure mix of argon gas, mercury vapour, and liquid mercury, and is coated on the inside with three different phosphorous substances. The electrodes provide a stream of electrons to the lamp and the ballast controls the current and voltage flowing into the assembly. The ballast, in general an electronic circuit, may be attached directly to the lamp, or may be remotely connected.
CFLs are compact and are ideal for use in homes, work areas, schools, workshops, etc. They are more energy-efficient than incandescent light bulbs using between one third and one fifth of the energy. They are sensitive to the ambient temperatures, just like the standard fluorescent lamps.
The life of a CFL is significantly shorter if it is used only for a few minutes at a time. Lab tests demonstrated that lifespan can be reduced down to 15% in the case of a 5 minute on/off cycle.
1.2.2.3 Low Pressure Sodium Lamps
A low pressure sodium lamp consists of a tube made of special sodium-resistant glass containing sodium and a neon-argon gas mixture. These lamps usually require 5 to 10 minutes to warm up. This light is basically monochromatic orange-yellow. This monochromatic light causes a dramatic lack of colour rendition: everything comes out in an orange-yellow version of black and white. Hence, low pressure sodium lamps are not suitable for use in homes, offices, workshops, etc. But they are widely used for street lighting purposes.
Low pressure sodium lamps are the most energy efficient visible light sources in common use. These lamps have luminous efficacies as high as 180 lumens per Watt, whereas a typical incandescent lamp has around 12 lumens per Watt and a standard fluorescent lamp has around 45 lumens per Watt.
1.2.3 Light Emitting Diodes (LEDs)
LEDs are semiconductor devices similar to p-n junction diodes, specially engineered to emit visible light of a particular wavelength, giving out a specific colour.
On supplying electrical power to an LED, electrons are made to “fall” from a high energy level to a low energy level inside the semiconductor material, releasing some energy, which is perceived as visible light.
LEDs are usually monochromatic and are very energy-efficient and therefore used extensively as indicator lamps on many electronic devices. Red, green, and orange LEDs as indicator lamps are usually well known. They are usually operated with no more than 20 – 70 mW of electrical power and not adequate for lighting purposes. However, the development of bright white LEDs advanced rapidly in recent years and is achieving fast access to the market. In 1999, the first 1 W white LEDs came on the market. In 2002, first 5 W models were available.
Parallel to this augmentation of the total power there has been also a dramatic increase in the luminous efficacies (see chapter 3.3) of LEDs during the past few years. Today the LEDs attain similar efficacy as CFLs and it is projected to increase further. In the last years, the price of bright white LEDs has fallen drastically and their availability increased considerably. Hence, LEDs are now used in many appliances like torches, bicycles and car lighting and are considered more and more for main stream lighting purposes.
LEDs have a longer life than the incandescent lamps and all types of gas discharge lamps. As they are solid-state devices, theoretically they offer operating periods of 100,000 to 150,000 hours. But they are the same time sensitive to ambient temperatures what caused some problems in first projects on LEDs as lighting devices in the tropics. A study by Lumitex Inc., U.S.A, indicates a substantial fall in the life of an LED, at elevated ambient temperatures (Fig. 4). Hence, special care must be taken to house LEDs properly, to ensure expected life. Life here implies, the time taken for the light output to fall to its 50% value, when compared to its initial light output.